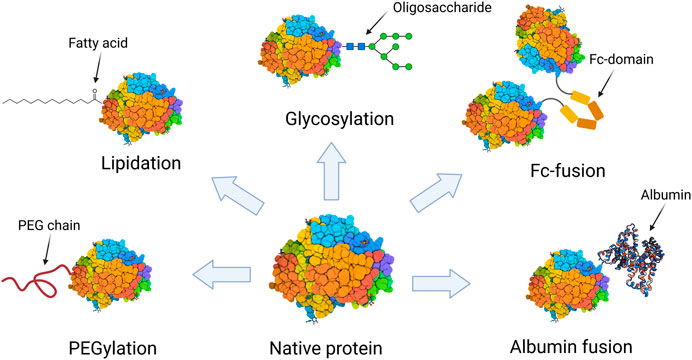
In the world of biotechnology and protein research, the purification of proteins is a critical step in ensuring their functionality, stability, and usability in various applications. One of the most important aspects of protein purification is maintaining the native state of the protein, ensuring that its structure and function are preserved throughout the process. Native purification is a technique specifically designed to achieve this goal, allowing researchers to obtain functional proteins that retain their biological activity.
In this article, we will explore what native purification is, its importance in protein research, and the methods used to achieve it while ensuring the integrity of the protein is maintained.
What is Native Purification?
Native purification refers to the process of isolating proteins from complex mixtures (such as cell lysates or tissue extracts) while preserving their natural (native) structure and biological activity. Unlike denaturing purification methods, which may involve harsh conditions like high temperatures or chemicals that unfold proteins, native purification aims to maintain the protein’s tertiary and quaternary structures, which are essential for its biological function.
The goal of native purification is not just to isolate the protein of interest but to ensure that it remains in its active form, enabling researchers to use it for further studies, such as enzyme assays, structural analysis, or therapeutic applications.
Importance of Native Purification
- Preserving Protein Functionality
Many proteins function only in their native state, meaning that their biological activity depends on their three-dimensional structure. If a protein is denatured (unfolded), it may lose its functional properties, making it unsuitable for research or therapeutic purposes. Native purification helps maintain this critical functionality. - Study of Protein-Protein Interactions
Many proteins function by interacting with other molecules, such as other proteins, DNA, or small ligands. Preserving the native structure during purification allows for the study of these interactions in their physiological context, which is crucial for understanding the underlying mechanisms of biological processes. - Therapeutic Applications
Purified native proteins are used in a variety of therapeutic applications, such as enzyme replacement therapy, antibody production, or vaccine development. Ensuring that the protein retains its natural structure and function is essential for its success in these applications. - Reducing Aggregation and Misfolding
During the purification process, proteins are at risk of aggregating or misfolding. Native purification methods are designed to minimize these risks by maintaining conditions that support protein stability.
Methods of Native Purification
Several techniques can be employed for native protein purification, each suited to different types of proteins and applications. Here, we outline some of the most commonly used methods:
1. Affinity Chromatography
Affinity chromatography is a powerful technique used to purify proteins based on their specific interactions with ligands or binding partners. In native purification, affinity chromatography can be performed under mild conditions, ensuring that the protein remains in its native form.
For example, a commonly used strategy is to attach a specific ligand, such as an antibody or substrate, to a column. When the protein mixture is passed through the column, the protein of interest binds to the ligand, while other proteins are washed away. The bound protein can then be eluted by altering the conditions (e.g., changing salt concentration or pH).
This method is highly specific and can be used to purify proteins without disrupting their native structure, making it ideal for maintaining protein activity.
2. Ion Exchange Chromatography
Ion exchange chromatography separates proteins based on their net charge. A resin with either a positive or negative charge is used to attract proteins of the opposite charge. In native purification, ion exchange chromatography is typically performed under gentle conditions (neutral pH, low salt concentrations) to prevent protein denaturation.
Proteins are eluted by increasing the ionic strength of the buffer, allowing for selective separation based on charge differences. This technique is useful for purifying proteins with distinct charge properties while preserving their native state.
3. Size Exclusion Chromatography (SEC)
Size exclusion chromatography, or gel filtration, separates proteins based on their size. During native purification, SEC is performed under mild conditions, often following other purification steps to remove contaminants or aggregated forms of the protein.
Larger proteins elute first, while smaller ones are retained in the pores of the column and elute later. This method helps remove smaller contaminants and aggregates, resulting in a purified protein that retains its native conformation and functional activity.
4. Hydrophobic Interaction Chromatography (HIC)
Hydrophobic interaction chromatography exploits the differences in hydrophobicity between proteins. Proteins are applied to a column under high salt conditions, which promote the hydrophobic interaction between the protein and the column matrix. As the salt concentration is gradually reduced, proteins are eluted based on their hydrophobicity.
HIC is an effective method for purifying membrane proteins or other proteins with hydrophobic regions, and it can be performed under conditions that preserve the native state of the protein, preventing denaturation.
5. Immobilized Metal Ion Affinity Chromatography (IMAC)
Immobilized metal ion affinity chromatography (IMAC) is a specialized form of affinity chromatography that utilizes metal ions, such as nickel or cobalt, which are bound to a column matrix. Proteins with metal-binding sites (such as His-tags) can bind to the metal ions, allowing for selective purification.
IMAC is particularly useful for recombinant proteins that have been tagged with a metal-binding domain, such as His-tagged proteins, and can be performed under native conditions to preserve the protein’s structure and function.
6. Ultrafiltration and Dialysis
Ultrafiltration and dialysis are techniques used to concentrate proteins and remove small contaminants, such as salts, buffer components, or small molecules. In native purification, these methods are employed to maintain protein integrity by using membranes with specific molecular weight cutoffs that retain larger proteins while allowing smaller molecules to pass through.
Both techniques are mild and do not interfere with the protein’s native structure, making them useful for polishing and concentrating purified proteins.
Challenges in Native Purification
While native purification offers significant advantages, it also presents challenges that must be carefully managed:
- Protein Aggregation
Proteins are prone to aggregation during purification, particularly if they are not handled gently. Aggregates may result in loss of function, making it crucial to optimize purification conditions to minimize aggregation. - Protein Stability
Some proteins are unstable or prone to denaturation under certain conditions. It is essential to optimize buffer compositions, pH, and temperature to maintain the protein’s stability during purification. - Protein Solubility
Some proteins, particularly membrane-bound proteins, may be insoluble in aqueous buffers and require special solubilization techniques. This can complicate the purification process while still maintaining native conditions.
Conclusion
Native purification is a vital process for obtaining functional, active proteins from complex mixtures. By preserving the protein’s natural structure and function, this approach ensures that the purified protein remains biologically relevant, making it ideal for a wide range of applications, including research, diagnostics, and therapeutic development.
The choice of purification technique—whether affinity chromatography, ion exchange, size exclusion, or others—depends on the specific protein and its characteristics. By carefully selecting the appropriate methods and optimizing conditions, researchers can achieve high purity and functional yield while preserving protein integrity, ultimately ensuring reliable and reproducible results in their studies and applications.